Figure: electron transport
Jump to navigation
Jump to search
Expression
- tissues with high rates of oxidative phosphorylation include:
- muscle, heart, & brain
Pathology
- the overall process is hugely inefficient
- the free energy for the formation of ATP from ADP is only +7.3 kcal/mole
- the 52.6 kcal/mole used from electron transfer to accomplish this leads to an efficiency of about 14%
- inefficient transfer of electrons leads to potential hazzards, especially with transfer of electrons to O2
- partial reduction products of O2, peroxides, hydroxyl radical & superoxide are cytotoxic & mutagenic
- thus mechanisms have evolved to scavenge these reactive oxygen species produced during oxidative phosphorylation
- considerable debate regarding the biological significance these reactive oxygen species surrounds the 'Free Radical Theory of Aging' proposed by Denham Harman in 1956.[2]
Biochemistry
- reduction of molecular oxygen at the inner mitochondrial membrane with production of ATP
- H+ gradient (higher pH with the mitochondrial matrix) provides driving force for F1 ATPase.
- oxidative phosphorylation or electron transport in eukaryotes occurs in mitochondria
- it takes place in the inner mitochondrial membrane, in contrast to the reactions of the citric acid cycle & fatty acid oxidation which occur in the mitochondrial matrix
- in oxidative phosphorylation, the electron transfer potential of NADH or FADH2 is converted into the phosphate-transfer potential of ATP
- the driving force of oxidative phosphorylation is the electron transfer potention of NADH or FADH2 relative to molecular oxygen (O2). For NADH this is:
1/2 O2 + 2 H+ + 2 e- -> H2O Eo = +0.82 V
NADH -> NAD+ + H+ + 2e- Eo = +0.32 V
----------------------------------------------------------------
1/2 O2 + NADH + H+ -> H2O + NAD+ E = +1.14 V
- the free energy is thus given by: G = nfE = (-2) (23.06) (1.14) = - 52.6 kcal/mole
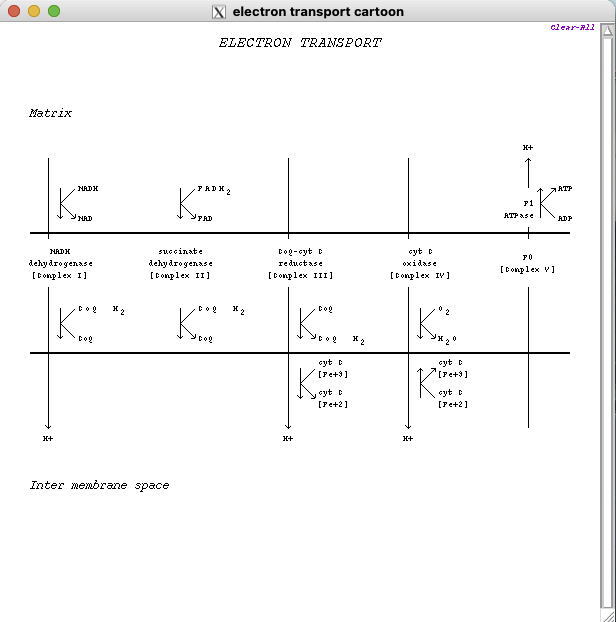
- electrons are transferred from NADH to O2 through a chain of 3 macromolecular complexes: complex I (NADH dehydrogenase), complex III (CoQ-cytochrome C reductase) & complex IV (cytochrome C oxidase)
- electron flow within these complexes leads to pumping of protons from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space.
- electrons are transferred from FADH2 to O2 through complex II (succinate dehydrogenase), complex III & complex IV
- electrons are shuttled between the complexes (complex I & complex II) & (complex II & complex III) by reduced coenzyme Q (CoQH2)
- cytochrome C shuttles electrons from complex III to complex IV
- complex IV catalyzes transfer of electrons from reduced cytochrome C to O2, the final electron acceptor
- 4 electrons are transferred to O2 to completely reduce it to H2O with concomitant transfer of H+ from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space
- complex V utilizes the proton-motive force generated by movement of protons across the inner mitochondrial membrane during electron transport to phosphorylate ATP
References
- ↑ Stryer Biochemistry WH Freeman & Co, New York, 1988 pg 398-424
- ↑ 2.0 2.1 Harman D, J Gerontol 11:298-300, 1956
- ↑ http://www.genome.ad.jp/kegg/pathway/map/map00190.html